July has been designated Juvenile Arthritis Awareness Month by the Arthritis Foundation. NIAMS estimates that about 294,000 American children under age 18 have arthritis or other rheumatic conditions. The Biophysical Society is taking this opportunity to highlight how advances in basic research contribute to our understanding of this disease. We spoke with BPS member Christine Beeton, Baylor College of Medicine, about her research related to rheumatoid arthritis, the most common form of arthritis in children. Her work focuses on targeting potassium channels for the treatment of chronic diseases including multiple sclerosis, rheumatoid arthritis, and type 1 myotonic dystrophy, and using antioxidant nanomaterials for the treatment of T lymphocyte-mediated autoimmune diseases (multiple sclerosis and rheumatoid arthritis).
What is the conn ection between your research and arthritis?
ection between your research and arthritis?
The term arthritis encompasses a number of diseases that affect joints; my focus is on rheumatoid arthritis (RA) that is characterized as a systemic inflammatory and chronic disease. In the last decades the role of resident joint cells, the fibroblast-like synoviocytes (FLS), has come to light in this disease. However, no therapeutic currently specifically targets these cells. We have identified KCa1.1 (BK, KCNMA1) as the major potassium channels at the plasma membrane of FLS isolated from patients with RA and have shown that blocking this channel inhibits pathogenic functions of FLS ex vivo and reduces the severity of two animal models of RA (Hu et al. J. Biol. Chem. 2012; Tanner et al. Arthritis Rheumatol. 2015). We are currently investigating the roles of these channels in the pathogenic functions of FLS and also as a potential target for therapy.
Why is your research important to those concerned about arthritis?
Current therapeutics for RA have significantly improve the wellbeing of the patients. However, most induce immunosuppression and therefore place the patients at risk for infections and tumor development. Our ability to target FLS has the potential of development effective treatments for RA that do not immunocompromised the patients. In addition, targeting both immune cells and FLS by combination therapy may offer the added benefit of using reduced amounts of drugs if the effect is synergistic.
How did you get into this area of research?
During my PhD in Immunology, I had the opportunity to study Kv1.3 channels in T lymphocytes, cells involved in autoimmune diseases. As a student my work focused on multiple sclerosis but as a postdoctoral fellow I extended this work to RA. When testing Kv1.3 blockers in animal models of RA and analyzing potassium channel expression in synovium biopsies from patients with RA I became interested in the potassium channel phenotype of other cells involved in RA. When I started my own laboratory I started a collaboration with Dr. Gulko, a rheumatologist now at Mount Sinai in New York, and focused my attention to FLS. This lead to the identification of KCa1.1 as the major potassium channel at the plasma membrane of these cells.
How long have you been working on it?
I started working on potassium channels in FLS from patients with RA in 2008, soon after starting my laboratory at Baylor College of Medicine.
Do you receive public funding for this work? If so, from what agency?
This work is not funded by the NIH but we have received funding from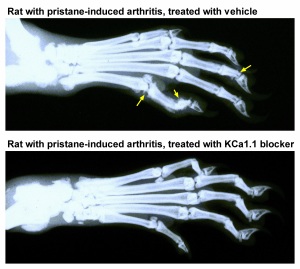 the Alkek Foundation for Medical Research and from Baylor College of Medicine.
the Alkek Foundation for Medical Research and from Baylor College of Medicine.
Have you had any surprise findings thus far?
Many. The happiest was to find out that FLS from patients with RA express one major potassium channel at their plasma membrane and not a combination of many channels, which would have made the work of identifying them and defining their roles a lot more complicated. The most puzzling was the finding, repeated many times, that blocking KCa1.1 induces a calcium transient in these cells. This really intrigued us and started us into studying the cell signaling downstream of the channel.
What is particularly interesting about the work from the perspective of other researchers?
Over the last few years, FLS have emerged as important players in the pathogenesis of RA. While many signaling molecules have been identified as regulators of various pathogenic functions of these cells, nothing was known about the expression and function of potassium channels. Our work therefore brings a new cell signaling regulator to light.
What is particularly interesting about the work from the perspective of the public?
Getting a better understanding of the different cell types that play a role in rheumatoid arthritis will help design better drugs to combat this disease. In addition, understanding the roles of KCa1.1 channels in the function of FLS during RA may help understand the roles of these and other potassium channels in other tissues and diseases.
Do you have a cool image you want to share with the blog post related to this research?
I am showing two cool images; one of an FLS, isolated from the joint of a patient with RA who had to undergo therapeutic joint surgery due to disease severity. The other shows X-ray images of the hind paws of rats with a model of RA induced by the injection of pristane. In the top X-ray, joint damage (yellow arrows) is visible; In contrast, the joints are much healthier in the paw of the rat that was treated with a KCa1.1 blocker for 21 days, starting at onset of clinical signs.